
Two of the essential amino acids, lysine and tryptophan, are poorly represented in most plant proteins. However, cysteine can partially meet the need for methionine (they both contain sulfur), and tyrosine can partially substitute for phenylalanine. These "essential" amino acids cannot be synthesized from other precursors. Humans must include adequate amounts of 9 amino acids in their diet. Tyrosine Tyr Y a phosphate or sulfate group can be covalently attached to its -OH Tryptophan Trp W scarce in most plant proteins Threonine Thr T carbohydrate can be covalently linked ("O-linked") to its -OH Serine Ser S carbohydrate can be covalently linked ("O-linked") to its -OH Lysine Lys K strongly basic and hydrophilic Glycine Gly G so small it is amphiphilic (can exist in any surroundings)
#Hydrophobic amino acids letter code free
Glutamic acid Glu E free carboxyl group makes it acidic and hydrophilic
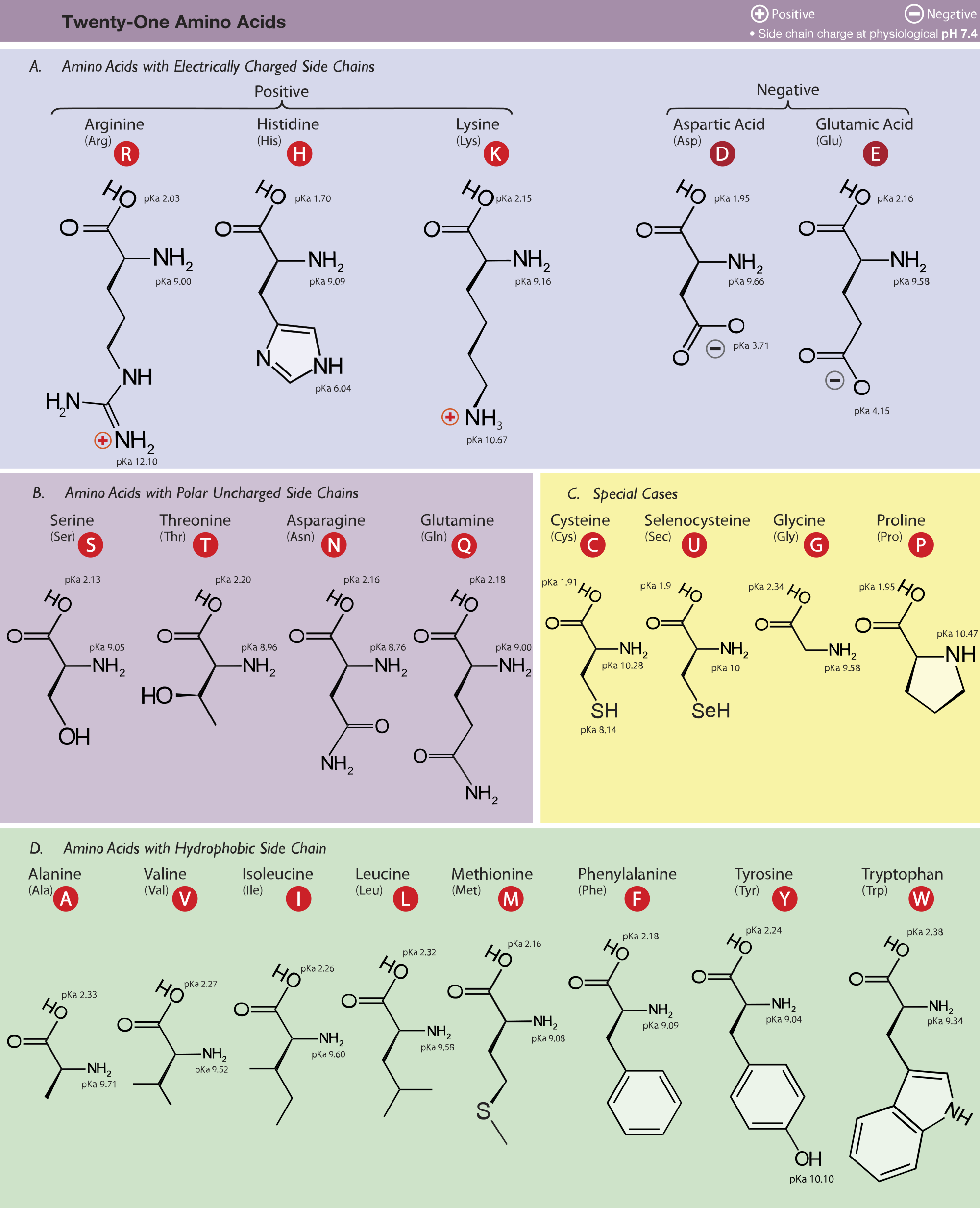
Click the name to see the structural formula) Alanine Ala A hydrophobicĪrginine Arg R free amino group makes it basic and hydrophilicĪsparagine Asn N carbohydrate can be covalently linked ("N-linked) to its -NHĪspartic acid Asp D free carboxyl group makes it acidic and hydrophilicĬysteine Cys C oxidation of their sulfhydryl (-SH) groups link 2 Cys (S-S)

(For each amino acid, both the three-letter and single-letter codes are given. It is the structure of the R group that determines which of the 20 it is and its special properties. This gives up a proton and is thus an acid (hence amino "acid") The shape and other properties of each protein is dictated by the precise sequence of amino acids in it.Įach amino acid consists of an alpha carbon atom to which is attached 20 different amino acids are used to synthesize proteins. Amino acids are the building blocks (monomers) of proteins.


 0 kommentar(er)
0 kommentar(er)
